Acid rain is nothing but normal rain or another type of precipitation that is acidic. Nowadays, acid rain is a key global environmental issue. In this article, every ins and out of acid rain is discussed. The defination of acid rain, main causes of acid rain, harmful effects of acid rain, formation of acid rain, ph of acid rain, acid rain control measures etc. have been discussed in this post in detail.
Contents
Table of Contents
Definition of acid rain:
Rainfall which is so acidic due to environmental pollution basically air pollution, it creates adverse effects to the aquatic ecosystem, plants, human health and as well as construction sites is called acid rain. In 1952 Scottish chemist Robert Angus Smith used the phrase acid rain while investigating rainwater in England and Scotland.
Due to the presence of CO2 in the atmosphere, which produces carbonic acid (H2CO3) by dissolving into cloud water, normal rainwater is always slightly acidic. So, virtually all rain in nature, before the Industrial Revolution, was acidic. Atmospheric carbon reacts with atmospheric water vapour or cloud water droplets to form carbonic acid (H2CO3). The chemical reaction is:
CO2 + H2O =H2CO3
Normal rainwater has a pH of around 5.6 because as it is mentioned earlier, rainwater naturally absorbs carbon dioxide in the air to form a mild carbonic acid. Water that is polluted and becomes too acidic will usually have a pH of below 5. The term ‘acid rain’ refers to the additional acidity in rain as a result of the emissions of sulfur dioxide(SO2 ) chiefly from coal-burning power and industrial plants and nitrogen oxides dominantly from vehicles. Because, of the presence of SO2, and NO2, pollutants in the atmosphere, the pH of the rainwater could be often as low as 2.4 and such type of precipitation with lower pH is called acid rain. Acid rain is basically a mixer of mainly H2SO4, and HNO3, where the ratio of these two acids may vary depending upon the relative quantities of oxides of Sulphur and Nitrogen emitted. H2SO4 is the major contributor (60-70%) to acid rain, while ranks HNO3 second (30-40%) and HCl third.
Sources of acid rain:
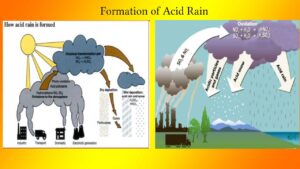
The primary causes of acid rain are SO2 and oxides of nitrogen (NOX). These pollutants originate from anthropogenic activities, chiefly from the combustion of burnable waste, fossil fuels in thermal power plants and automobiles. These sulphur dioxides (SO,) and oxides of nitrogen (NOx) interact with reactants present in the atmosphere and result in acid deposition. Natural sources also generate sulphur pollutants, like oceans and to a smaller extent from volcanic eruptions. The anthropogenic sources of SO2 emissions are the burning of coal and petroleum and various industrial processes, Other sources include the smelting of iron and other metallic (Zn and Cu) ores manufacture of sulphuric acids and the operation of acid concentration in the petroleum industry. The concentration of NOx in the atmosphere is small in comparison to SO2, but its contribution to the production of acid rain is increasing day by day. Main natural sources of NOx include lightning, volcanic eruptions and biological processes particularly microbial activity. Anthropogenic sources are power stations, vehicle exhausts and industrial emissions.
Chemical reactions in acid rain formation:
The interaction of SO2, and NOx with atmospheric reactants and their chemical reaction results in the formation of acid rain. When the constituents of sulphur and nitrogen released into the atmosphere from smoke stakes of power plants and industrial establishments, molecules of SO2, and NOx are caught up in the prevailing winds, and soon after they interact in the presence of sunlight with water vapours to form sulphuric acid and nitric acid mists. These acids remain in the vapour phase under prevalent high-temperature conditions. Condensation takes in form of aerosol droplets when the temperature falls, which owing to the presence of un-burnt carbon particles will be black, acidic and carbonaceous in nature. This matter is called “acid smut”. The presence of oxidizing agents in the atmosphere and the characteristics of the reaction that takes place in the atmosphere affects the rate of acid generation. Acid precipitation, popularly known as acid rain, is apparently due to the oxidation of nitrogen and sulfur-containing gases that dissolve in the water vapour of the atmosphere to form nitric and sulfuric acids.
The acid-forming reaction of sulfur:
Since coal is reached in sulfur, as it is burned oxidation takes place and SO2 formed.
S + O2=SO2
SO2 is released into the atmosphere from the smokestacks and dispersed by the prevailing wind, and slowly oxidized to form sulfate at the ordinary temperature.
2 SO2 + O2 = 2SO32-
SO32- + H2O = H2SO4
SO2 + H2O = H2SO3 (H+ HSO3–)
H2SO3–+ O3 = SO42+ + H+ + O2
H2O2 + HSO3– =HSO4– +H2O (Acid mist)
Acid forming reaction of NOx:
N2 + O2 = 2NO
2NO + O2 = 2NO2
4NO2 + O2 + 2H2O = 4HNO3
O3 + NO2 = N2O5
N2O5 + H2O = 2HNO3
The adverse effect of acid rain:
Some of the acidic pollutants do not rise very far into the air and soon after they return to the earth and may fall directly onto the surface of plants, buildings, water or soil, which is known as dry deposition. These deposits turn into acidic droplets if dew or rain comes into contact with them. If the pollutants fall into water then they will dissolve and form an acidic liquid. The longer the sulphur dioxide and nitrogen oxides remain in the air the more likely they are to react with moisture in the air and create sulphuric and nitric acids and fall out from the atmosphere as rain. This is called wet deposition. Acid rain has had clearly demonstrable negative effects on surface waters, soil, flora, fauna and atmospheric visibility. Some specific effects are as follows:
Effects of acid rain on soil:
Plants entirely depend on soil for their nutrient and water supply. Acid rain results in acidification of soil, leading to higher cations exchange between hydrogen ion and nutrient cations like potassium (K), magnesium (Mg) and calcium (Ca) in the soil. These nutrient cations rapidly leached out in soil solution along with sulphate from acid input. Soon after acid-induced leaching leads to nutrient-deficient soils and this loss of soil fertility results in lowered growth of plants. Nutrient cycling and decomposition rate of organic matter in the soil is also negatively affected by acidification of soil. Strong acidifications slow down the decomposition of the litter of spruce, pine, birch and other cellulose-rich materials.

Effects of acid rain on the aquatic ecosystem:
Water bodies become acidic due to acid rain. Acidification more observed in streams and lakes due to acid rain, because water bodies have less potentiality of buffering of acid inputs than soils and plants. All components of an aquatic ecosystem like phytoplankton, amphibian, invertebrate, etc. are affected by acid rain. Due to the acidification of the aquatic ecosystem, fishes showed increases in mortality rate, reduced growth rate, skeletal deformities, reproductive failure, and increased uptake of heavy metals. The number of snails and phytoplankton also getting down if pH falls below 5.5 when pH is then 5.2, Snail disappeared; at pH 5.0, zooplankton disappeared; and below pH 4.0, stocks of all fish species declined rapidly because embryos failed to mature at this level of acidity.

Effects of acid rain on plants:
The effect of acid depositions on plants basically arises in the form of damage to foliage and roots. Damage to the tissue of roots and foliage of plant cause reduced canopy cover, crown dieback and whole tree death. The germination of some plants specifically coniferous species like Norway spruce, Scots Pine and silver birch seeds are affected by acid rain. Anatomical alterations in the leaves of tropical species, seedlings and saplings and; various physiological processes like photosynthetic rate, stomatal conductance, etc. and morphological characteristics of plants were found to be negatively affected by Acid rain. Plants community like algae, fungi and lichen as well as various microorganisms and microbial processes within the soil get affected due to modification in soil properties by acid rain.

Effects of acid rain on materials and buildings:
The impact of acid deposition on stone monuments made of marble and limestone and on building materials containing large amounts of carbonate has been recognized for over a century and many studies have addressed the effect of acid wet deposition on stone materials of historic buildings and monuments like Tajmahal in India. High buildings made of sandstone, limestone and marble in urban areas have damaged due to exposure to cloud water with high acidity for a long time. Calcium carbonate is the common constituent of these materials, which reacts with the sulfur present in dry deposition and form soluble calcium sulfate and resulting acids washed off from the surface of the stone when it rains and damages the world’s cultural heritage, ancient monuments, historic buildings, Sculptures, ornaments and other important cultural objects.

Effects of acid rain on human health:
Though acid rain is the invisible form of pollution, it has some indirect effects on human health like damage to humans by contact with materials that have themselves been affected by acidification like food and water supplies. SO2 causes more adverse impacts to human health in gas and aerosol forms. SO2 concentration above 1.6 ppm causes detectable breathing difficulty and eye irritation. SO2 is much more toxic and damaging in combination with aerosols, and mists, and suspended smoke; because of this mixture the mixture of chemicals form finer suspensions that penetrate the lungs further than the gas alone. Among the indirect effect of acid rain on human health involves toxic heavy metals pollution of soil and water, because these are liberated from the soil due to soil acidification. Such mobilized contaminants like Al, Cd, Zn, Pb, Hg, Mn and Fe are dissolved in soil and water make their way to groundwater that is drunk by humans and contaminate food like Fish, meat, and vegetables eaten by humans.
Control measures of acid rain:
Acid rain is caused by mainly two pollutants sulfur dioxide (SO2) and nitrogen oxides (NOx) which are released into the atmosphere due to fossil fuel burning. So, control acid rain basically refers to the control of these pollutants:
- A common method for the reduction of SO2, emissions involves burning of coal containing less sulfur, washing the coal, and using devices called scrubbers to chemically remove the SO2 from the gases leaving the smokestack. Nowadays, power plants are switching from burning of coal to natural gas, because it creates much less SO2. Power plants can use technologies that rely on renewable energy instead of fossil fuels.
- Throughout the world, catalytic converters are installed in vehicles to reduce NOx emissions.
- Use alternative energy sources like nuclear power, hydropower, wind energy, geothermal energy, and solar energy for power plants and vehicles also reduce the emission of acidic pollutants.
- There are also alternative energies, such as natural gas, batteries, and fuel cells, available to power automobiles to reduce the emission of acidic pollutants.
- Restoring a damaged environment can also be served as a control measure of acid rain. For example, limestone or lime can be added to acidic lakes to reduce acidity. Many chemicals like caustic soda, sodium carbonate, slacked lime, and limestone is most popular for raising the pH of acidified water.
Acid rain is one of the key global environmental issues. If you still not clear about acid rain feel free to comment below. Besides, you may watch this video about acid rain for a better understanding.